Telomere Dynamics in Companion Animals: What Do We Really Know?
Telomeres are protective nucleoprotein structures located at the ends of linear chromosomes. They consist of repetitive DNA sequences and associated proteins that stabilize chromosomal integrity during cell division. Across species, telomere dynamics have been widely studied as one measurable feature associated with biological aging.
In companion animals, particularly dogs, telomere biology has attracted growing interest within comparative aging research. However, interpretation of telomere measurements requires careful scientific restraint.
This article examines what is currently understood — and what remains uncertain — about telomeres in companion animals.
What Are Telomeres?
During cellular replication, DNA polymerase cannot fully replicate the terminal ends of linear DNA strands. As a result, telomeres gradually shorten with each cell division. This progressive shortening is often described as a marker of cellular replicative history.
When telomeres reach a critically short length, cells may enter replicative senescence — a state in which further division is limited. This process contributes to tissue-level changes observed in aging organisms.
Importantly, telomere shortening is not synonymous with organismal aging itself. It represents one component of a broader and multifactorial biological process.
Telomere Biology in Dogs
Dogs share many fundamental biological aging processes with humans, including telomere shortening over time. Several studies have examined telomere length in canine leukocytes and other tissues, observing associations between chronological age and telomere attrition.
However, notable distinctions exist:
-
Telomere length varies between breeds
-
Genetic background influences baseline telomere measurements
-
Environmental factors may contribute to variability
-
Measurement techniques differ across studies
Because of these factors, cross-study comparisons require caution.
Breed size and lifespan variability in dogs add additional complexity.
Large breeds often exhibit shorter average lifespans compared to smaller breeds, but direct causative links between telomere length and breed-specific longevity remain under investigation.
Measurement Considerations
Telomere length in dogs is commonly assessed using:
-
Each method carries inherent variability and technical limitations. Differences in laboratory protocols, sample handling, and tissue selection can influence reported values.
Furthermore, telomere measurements are typically obtained from peripheral blood cells. These reflect leukocyte populations, which undergo continuous turnover. Telomere length in blood does not necessarily represent telomere dynamics in all tissues.
Therefore, telomere length should be interpreted as a biological indicator within a specific measurement context, not as a universal aging metric.
Telomeres and Biomarkers of Aging
In aging research, telomere length is often categorized as a biomarker associated with cellular aging processes. Biomarkers provide measurable biological signals under defined conditions.
However, it is critical to distinguish between:
-
Changes in telomere-associated parameters may reflect shifts in cellular turnover, oxidative stress exposure, or replicative history. They do not independently demonstrate lifespan extension, disease prevention, or functional improvement.
The relationship between telomere dynamics and organismal aging remains complex and incompletely defined.
Telomere Dynamics and Cellular Turnover
Certain tissues — including immune cells and gastrointestinal epithelium — require continuous renewal. In these systems, telomere shortening may occur more rapidly due to repeated replication cycles.
In companion animals, immune function is of particular interest in aging research. Age-associated immune modulation has been described across species, and telomere dynamics in immune cells may provide one measurable parameter within this context.
Still, telomere length represents only one dimension of cellular maintenance. Mitochondrial function, DNA repair capacity, proteostasis, and metabolic regulation all contribute to aging biology.
A comprehensive understanding requires integrating multiple biomarkers rather than relying on a single metric.
Nutritional Context
Within the broader field of geroscience, nutritional research has explored how dietary factors interact with cellular processes associated with aging. In high-turnover tissues, cellular replication depends on coordinated metabolic pathways and substrate availability for DNA and RNA synthesis.
While telomere dynamics are influenced by cellular replication and oxidative stress, no dietary intervention can be assumed to directly determine telomere length independent of broader biological systems.
Nutritional research in this area must therefore be framed within:
-
Substrate support differs fundamentally from direct modulation of telomerase activity or genetic mechanisms.
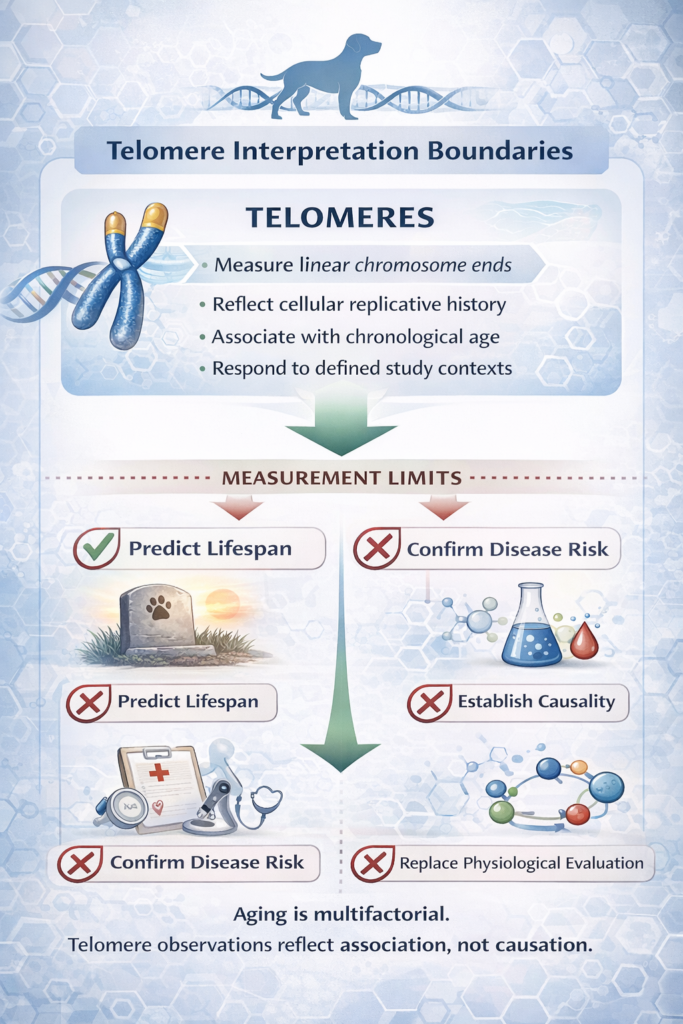
What Telomeres Can — and Cannot — Tell Us
Telomere measurements in companion animals may provide insight into:
-
They cannot:
-
Scientific rigor requires acknowledging both their value and their limitations.
Moving Forward in Canine Aging Research
As aging biology continues to evolve, telomere research in companion animals contributes to a growing body of comparative knowledge. Dogs provide a valuable model for understanding shared aging mechanisms between species.
However, responsible communication is essential. Telomere data must be interpreted proportionally to study design and methodological constraints.
Aging is multifactorial. Telomeres represent one measurable feature within a complex biological landscape that includes metabolic regulation, immune modulation, mitochondrial dynamics, and environmental influences.
Advancing canine geronutrition requires integrating these dimensions carefully — not isolating any single parameter beyond its evidentiary scope.
Interpretation Statement
The information presented reflects current understanding of telomere biology within defined research contexts. Biomarker observations do not independently imply lifespan extension or clinical outcomes. Findings should be interpreted within the parameters of experimental design and measurement methodology.
